The majority of our work for private-sector clients is confidential and some of the projects and reports we have completed for not-for-profit clients are for internal use only. Whenever possible, we encourage our clients to publish in the public domain at least some of the work to which we have contributed. These are the areas where we have done most work recently.
Preferred product characteristics - for the World Health Organization: Herpes simple virus vaccines (published 14 May 2019).
Meeting reports - for the World Health Organization: Gonnorhoea vaccines (draft with client) | Herpes simple virus vaccines | Immunization in older adults | Influenza virus vaccines - broadly protective/universal (2016; 2014) | Product Development for Vaccines Advisory Committee (PDVAC) (2018 and 2019 - drafts with client).
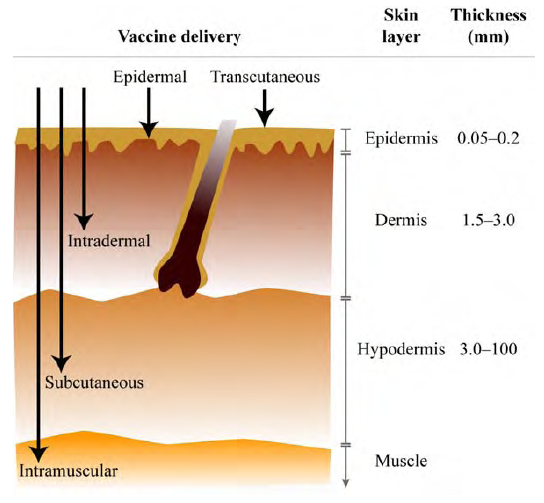
Intradermal (ID) delivery of vaccines
Since 2009, we have completed several reports on aspects of intradermal (ID) delivery of vaccines. Our work originated from initial interest from Project Optimize in whether ID delivery and dose-sparing of vaccines could have benefits in low- and middle-income countries, such as reducing vaccine costs and reducing vaccine cold-chain volume. We started with a review of the preclinical and clinical evidence, which was presented to Project Optimize to influence their strategy.
A figure from the first ID report
Report: J Hickling, R Jones. 'Intradermal delivery of vaccines' - a review of the literature and the potential for development for use in low and middle-income countries - 27 August 2009 (On PATH website).
The project was commissioned by PATH, the Disposable Syringe Jet Injector Project and Project Optimize (Immunization Systems and Technologies for Tomorrow), a collaborative project between PATH and the World Health Organization (WHO).
To advise the work of the Disposable Syringe Jet Injector Project team, we followed this with a more in-depth intradermal device R&D strategy, based on the initial landscape analysis. This was for internal use only but influenced their clinical development strategy, including with yellow fever (YF) vaccine (see below).
A related (short) Policy and Practices article was then published in the Bulletin of the World Health Organization, which updated the original landscape report and aimed to promote its use in the global health community. This has been widely cited.
Article: JK Hickling, KR Jones, M Friede, D Zehrung, D Chen & D Kristensen (2011). Intradermal delivery of vaccines: potential benefits and current challenges. Bull World Health Organ 2011; 89: 221–226 (Policy and Practice article on WHO website).
A presentation by Working in Tandem provided a summary the current status of dose-sparing data. The presentation also reviewed why ID administration of vaccines and dose-sparing need to be considered on a vaccine-specific basis and also considered from the different perspectives of the various stakeholders involved in vaccine supply and delivery.
Presentation: Cutaneous delivery of vaccines and dose-sparing: vaccine-specific issues. Presented at Vaccine Delivery and Stabilization: Improving the Reach of Vaccines, 9 September 2013, Boston, MA USA.
Yellow fever - dose sparing
This project, working closely with WHO, was based on a comprehensive literature review, attendance at an international meeting on flavivirus vaccines, data from WHO and interviews with experts from academia, clinical research and vaccine manufacturers.
The aim of the review was to see whether dose-sparing, which can be achieved by intradermal (ID) delivery of vaccines (i.e. injection into the skin), could improve the availability of yellow fever (YF) vaccine, an important vaccine that is often in limited supply.
We compared a dose-sparing strategy, with other alternatives to improving vaccine supply, and estimated likely impact. We also outlined a clinical development programme to evaluate this approach.
A figure from the yellow fever report
Report: J Hickling, R Jones. Yellow Fever Vaccination: The Potential of Dose-Sparing to Increase Vaccine Supply and Availability. 18 April 2013. (Full report on PATH website).
The project was commissioned with funds provided by the Bill & Melinda Gates Foundation through the Disposable Syringe Jet Injector project within the Delivery portfolio of the Vaccine Technologies Group at PATH.
BCG
We carried out a comprehensive review of issues arising from BCG vaccination and the evidence for a need, or desire, to replace the Mantoux method with novel intradermal delivery devices. This vaccine is very widely used, mostly in young (wriggling) infants and, as such, it could be a stable market for novel intradermal devices. This report was used internally by PATH. It is a fascinating and complex area, with implications for the use of this route for other vaccines.
Polio vaccines
We have completed several projects about polio vaccines and in particular inactivated poliovirus vaccine (IPV).Our first work started with a rapid analysis on 'use of vaccines for polio outbreaks in the UK', for the UK Department of Health (DH); this is now quite outdated and is no longer on the UK's DH website. For PATH, we co-authored a report on strategies to reduce costs of vaccination with IPV:
Report: J Hickling, R Jones, Nundy N. Improving the affordability of inactivated poliovirus vaccines (IPV) for use in low- and middle-income countries - An economic analysis of strategies to reduce the cost of routine IPV immunization 20 April 2010 (full report on Polioeradication website).
This work was supported by funding from the Bill & Melinda Gates Foundation. We have also contributed to various incremental cost analyses for dose-sparing of IPV, using intradermal devices and in 2013 - 2014, we are contributing to a follow-up report on intradermal IPV use within and after the Polio Endgame Plan, again for PATH.
Thermostability of vaccines
Since 2006, we worked with PATH and also technology developers on a number of projects related to improving the thermostability of vaccines. There are two main areas of interest (a) protecting vaccines from damage above 'fridge temperature' and (b) protecting some vaccines from damage due to 'freezing'. Project Optimize and others have done some very interesting work on using existing vaccines in 'controlled temperature conditions', which could allow much simpler transport and storage, especially in outreach settings. Re-formulating vaccines using thermostabilising technologies is more complicated, but could be 'transformative' to the ways vaccines are used in the future. Initially we carried out some project management work for PATH working with contractors in Europe that provide spray-drying services and liaising with vaccine manufacturers. Some of the results of this work have been published.
Article: Chen, D, S Kapre, A Goel, K Suresh, S Beri, J Hickling, J Jensen, et al. 2010. “Thermostable Formulations of a Hepatitis B Vaccine and a Meningitis A Polysaccharide Conjugate Vaccine Produced by a Spray Drying Method.” Vaccine 28 (31): 5093–99. doi:10.1016/j.vaccine.2010.04.112. (PubMed).
We also compiled tables summarizing stability data for licensed and investigational formulations of vaccines. These have been widely used. Although we update these tables on request, they are not 'living documents' and this is an evolving area of research so please be aware that some of the information might be out of date.
Tables: 'Summary of stability data for licensed vaccines'; and 'Summary of stability data for investigational formulations of vaccines'. Produced by Working in Tandem Ltd for PATH Vaccine and Pharmaceutical Technologies Group (on PATH website).
Vaccines and delivery methods
We have done several projects on pipelines of novel vaccines for various diseases and also for novel delivery devices. Most of these have been for private sector clients and are confidential. One of our early reports in this area was a broad overview of the landscape of vaccines and delivery devices for global health.
Report: Landscape analysis on Trends in Vaccine Availability and Novel Vaccine Delivery Technologies: 2008–2025. 2008 (on PATH website).
We have also authored and co-authored more-detailed reports, used internally within PATH, on the landscape of intranasal and oral vaccine delivery devices that could be used with specific vaccines of interest to PATH. We have an active interest in new technologies for vaccine formulation and delivery, especially those that could offer multiple benefits to users and purchasers. One example of such a potentially 'transformative' technology is the microneedle array (skin) patch. We work with the advisory board of a company developing a technology in this area.
We also helped a developer of safer, easier to use intradermal syringes, looking for applications and clinical collaborators.
Influenza vaccines
We have completed several projects on various aspects of influenza vaccination, especially for pandemic flu, for the UK Department of Health (for internal use only). We also completed a short project, with WHO, on approaches to manufacturing influenza vaccines and their implications for pandemic preparedness:
Report: A review of production technologies for influenza virus vaccines, and their suitability for deployment in developing countries for influenza pandemic preparedness for World Health Organisation, 20 December 2006 (no longer available on the WHO website but available here).
We have also contributed to projects, for internal use only, about delivery of live attenuated influenza vaccine for global health, especially pandemic flu.
Product development
We like to work with vaccine and product developers, academic or private-sector, at all stages of development, from initial thoughts about possible applications for their technologies, helping them develop 'target product profiles', guiding and advising on preclinical and clinical research, estimating possible demand, seeking funding, finding clinical collaborators, co-authoring or reviewing clinical protocols, and reviewing and writing-up data. We have also done some interim project management.
Technical due diligence
We have done many rapid analyses of individual technologies and early-stage companies for venture capital funders. Some of these have led to us providing follow-on assistance for the successful recipients of the investment.
Writing, editing and 'information design'
Before 'working in tandem' with Julian, Rebecca spent several years in academic publishing and also working in communications more generally... researching, writing, editing, illustrating, for molecular medicine journals, patient information, technical documents and websites. A set of 'transferable skills' that we use everyday in our other projects but also, from time to time, use on other people's projects.
Last updated: 15 November 2023